Schedule a Call Back
search Result

REC Launches Rs 50.71 Mn Mobile Medical Units in Rajasthan
Each MMU is equipped with GPS-enabled tracking systems to ensure efficient monitoring and delivery of healthcare services.Read more

The Growing Threat of Heat Stress in the Workplace
Extreme heat puts millions of workers at serious risk globally. Suresh Tanwar, Senior Head of Audit and Consultancy, British Safety Council, India, highlights the growing threat of heat stress in the workplace in India, where workers face extreme heat conditions with limited protection.Read more

Altem organises 3D printing workshop for innovations in medicine and healthcare
The workshop offered a diverse lineup of sessions focusing on 3D printing applications in surgical education, neurosurgery, orthopaedics, and biomedical engineering.Read more
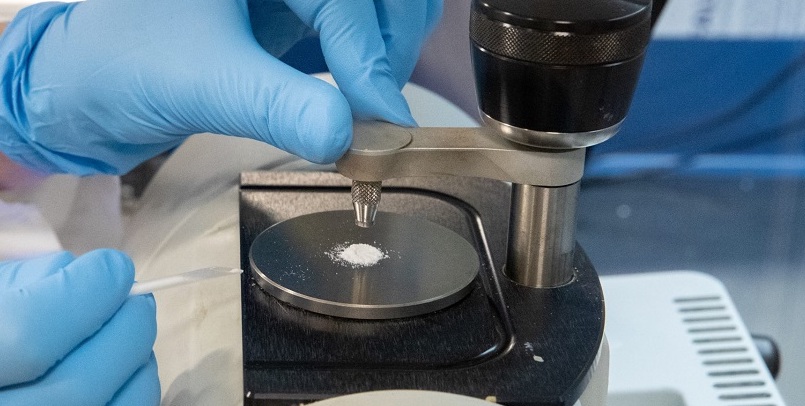
IISc discovers method to break down biofilm barriers using cow's gut enzyme
To break down the biofilm, the IISc team focused on using enzymes that degrade polysaccharides. Read more

Role of AI and automation in quality control in the pharmaceutical industry
In recent months, the Indian pharma industry, accounting for 20% of the global generic exports, has been facing problems related to quality from global & local regulators. By leveraging AI and automation, the industry can not only overcome quality challenges, but can also help it reach the target of $ 130 billion by 2030, says Sanskriti Ramachandran.Read more

AI can detect anomalies during production: Bharat Shah
In this exclusive interview with Sanskriti Ramachandran, Bharat Shah of SK Healthcare, delves into the role of ai and automation in the pharma industry. Read more


Tata Steel awarded worldsteel Safety and Health Excellence Recognition 2024
Tata Steel had also bagged the worldsteel Safety and Heath Excellence Recognition 2023 for its real-time visualisation of risk movement.Read more

Avians High-speed Clean Room Doors for Precision and Sterility
The company’s high-speed clean room doors have emerged as a critical asset in in healthcare and research laboratories.Read more
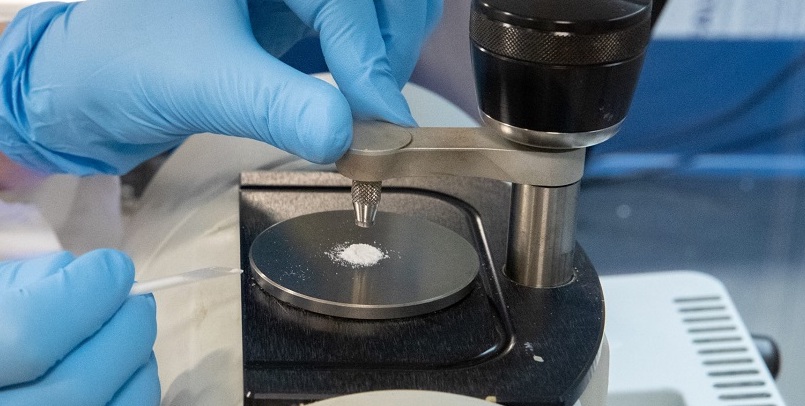
Manufacturing approval granted for three indigenous Mpox testing kits
These RT-PCR kits, which utilise fluid samples from pox rashes to detect the virus, were validated by the Indian Council of Medical Research (ICMR). Read more

Pharma firms ask for December 2026 as extension for implementation of rules
Schedule M of the Drugs and Cosmetics Rules, 1945, outlines good manufacturing practices (GMP) for pharmaceutical products, including a prompt recall system for defective products.Read more













